Leading Indian Medical Equipment Export Company
Axion Nova Exports LLP is a premier ISO 13485:2016 certified exporter of medical and surgical disposables based in Ahmedabad, Gujarat, India. Established with the vision of bridging the gap between quality Indian medical manufacturing and global healthcare needs, we have grown to become a trusted B2B partner for hospitals, distributors, procurement agencies, and healthcare facilities across 30+ countries in Asia, Africa, Middle East, and Europe.
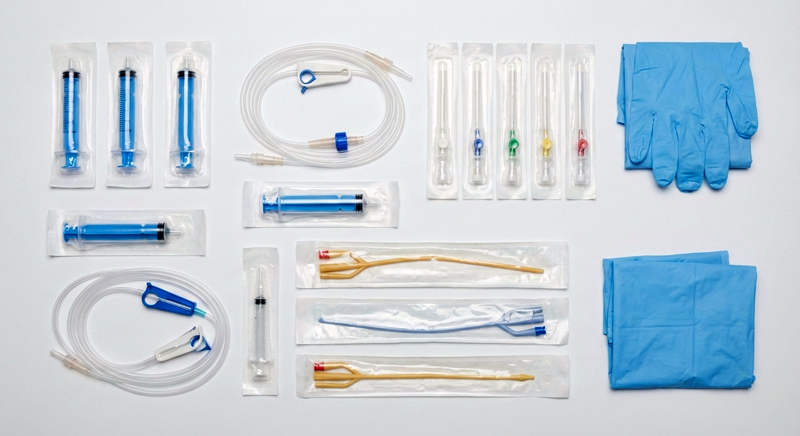
Our comprehensive export portfolio includes sterile medical disposables (surgical gloves, masks, gowns), surgical equipment (instruments, kits), hospital consumables (IV sets, catheters, urine bags), and diagnostic supplies — all manufactured to the highest international quality standards including ISO 13485:2016 Medical Device Quality Management, CE marking for European markets, and CDSCO approval for Indian regulatory compliance.
With over 5,000 successful international export shipments and a proven track record of consistent quality and on-time delivery, we specialize in providing complete export documentation, international regulatory compliance support, customs clearance assistance, and end-to-end logistics solutions for bulk medical product exports from India to global markets.
Our Mission
To provide healthcare facilities worldwide with access to premium-quality, internationally certified medical disposables manufactured in India, ensuring patient safety through rigorous quality control and transparent export processes.
Our Vision
To be recognized as the most reliable medical exports partner from India, setting industry benchmarks for quality, compliance, and customer service while contributing to global healthcare accessibility.